What Animals Are Known To Be Intermediate Hosts For Toxoplasma
- Review
- Open Access
- Published:
The life-bike of Toxoplasma gondii reviewed using animations
Parasites & Vectors volume 13, Article number:588 (2020) Cite this article
Abstract
Toxoplasma gondii is a protozoan parasite that is the causative agent of toxoplasmosis, an infection with loftier prevalence worldwide. Most of the infected individuals are either asymptomatic or have mild symptoms, merely T. gondii can cause severe neurologic damage and even death of the fetus when acquired during pregnancy. Information technology is likewise a serious condition in immunodeficient patients. The life-cycle of T. gondii is circuitous, with more than than one infective form and several transmission pathways. In two animated videos, we depict the main aspects of this bicycle, raising questions nearly poorly or unknown problems of T. gondii biology. Original plates, based on electron microscope observations, are besides available for teachers, students and researchers. The main goal of this review is to provide a source of learning on the fundamental aspects of T. gondii biology to students and teachers contributing for better knowledge and command on this important parasite, and unique jail cell model. In improver, drawings and videos betoken to notwithstanding unclear aspects of T. gondii lytic bike that may stimulate further studies.
Graphical Abstruse
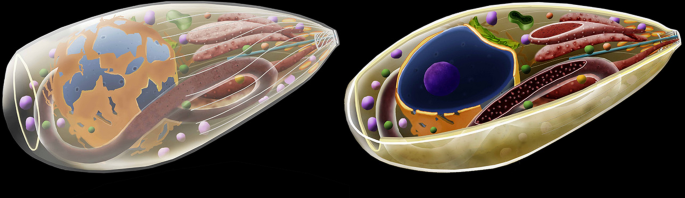
Background
Toxoplasma gondii is the causative amanuensis of toxoplasmosis that is a zoonosis of significant medical and veterinary importance and is transmitted by several pathways. Marked advances regarding the control of several infectious diseases acquired by parasitic protozoa have taken place in the terminal decades, peculiarly those that spend office of their life-cycle inside host cells. Withal, the epidemiological control and evolution of new chemotherapeutic agents with low toxicity and high specificity continue to institute keen challenges. Some of these diseases are restricted to specific areas of the world, as in the case of Chagas disease. Others, like toxoplasmosis, are widely distributed throughout the globe [1]. Indeed, T. gondii, a fellow member of the phylum Apicomplexa, developed the ability to infect well-nigh whatsoever prison cell type of mammals and birds [2, 3]. In the USA, information technology was estimated that eleven% of the population aged six years and older have been infected with T. gondii. In several countries throughout the world, it has been shown that more than than 60% of the people have been infected with T. gondii [4]. In some geographical areas (e.m. Brazil), upwardly to 60% of the population is seropositive for T. gondii antigens [5]. The environmental weather condition and dietary habits can touch on infection rates. For example, the ingestion of raw or undercooked meat is associated with T. gondii transmission, and pig and sheep meat are more decumbent to contain tissue cysts than cattle [6,7,8]. Contamination by oocysts excreted with true cat carrion does not necessarily involve contact with the cat itself. Pet cats are less subject to becoming contaminated (and produce oocysts) than cats living on the street or rural areas [1, five, 9, 10].
The genus Toxoplasma contains only one species, T. gondii, that can be grouped into genotypes (types I, II and III, XII and the haplotypes Ten and A). Some are restricted to wild animals.
It is e'er of import to review and disseminate the current noesis on T. gondii and other parasites not just to higher, undergraduate and high school students, but too to the general population, every bit office of an try to eradicate, or at to the lowest degree control or foreclose the affliction burden of toxoplasmosis, particularly among women at reproductive age, since they are the main group at risk.
We have previously developed educational material to teach fundamental biological aspects on Chagas disease and leishmaniasis caused, respectively, past Trypanosoma cruzi and Leishmania spp., with emphasis on dynamic processes and iii-dimensional views [11, 12].
Available fabric
Here we present a review and innovative multimedia materials showing bones aspects of the life-cycle of the protozoan T. gondii and its morphology, based on electron microscopy observations of the diverse developmental stages of T. gondii. In add-on to the PowerPoint slide show that is bachelor at (Additional file 23: Slideshow), we produced videos that can exist visualized in Boosted files 21 and 22: Videos SV1 and SV2 or in https://pesquisa.biof.ufrj.br/biologia-celular-parasitologia/luchm/ . The 3D models and animations were based on our group's information obtained over the terminal 30 years using video-microscopy and scanning and transmission electron microscopy. They evidence various aspects of the structural organization of the different developmental stages of T. gondii [xiii,14,fifteen,16,17,xviii,19,20,21,22,23]. Our assay besides considered the contributions of several other groups that accept provided relevant contributions to the field [24,25,26]. All animations and images were produced using software such as 3D Max (Autodesk Inc., San Rafael, CA. USA; 94903), AfterEffects, Photoshop and Illustrator (all three from Adobe; www.adobe.com).
The evolution of these videos raised questions that led to speculations on the dynamics of some still poorly characterized biological processes in an attempt to stimulate farther research to confirm or invalidate some hypotheses.
Biological cycle (transmission pathways)
It is well known that T. gondii infects hosts that include terrestrial and aquatic mammals and birds. These animals are considered intermediate hosts because only asexual stages occur in them (Fig. 1). The sexual stages are seen only in the members of the family Felidae, including the domestic cat [3, 27, 28]. Therefore, they are considered to be definitive hosts. Even so, it has been recently reported that inhibition of murine-delta-six-desaturase activity in the mouse intestine and supplementation of the nutrition of mice with linoleic acid allowed T. gondii sexual development in the intestinal cells of mice [29].
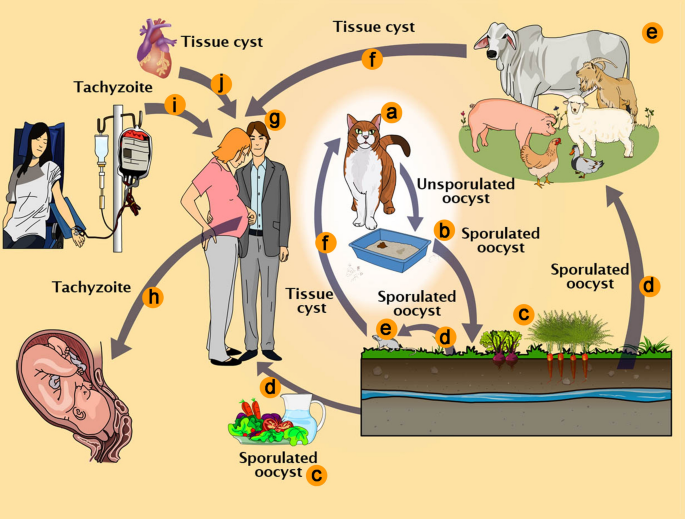
Toxoplasma gondii pathways of transmission. a Feline definitive host (cat). b Unsporulated oocysts in cat carrion. c Nutrient contaminated with sporulated oocysts. d Oocysts may be ingested by intermediate hosts via water or raw vegetables. e Intermediate hosts (e.g. cattle, sheep, poultry and swine). f Ingestion of tissue cysts in uncooked meat. g Intermediate hosts (humans). h Tachyzoites transmitted through the placenta to the foetus. i Transmission past claret transfusion and organ transplant (j)
During the life-bike of T. gondii, three developmental stages can infect cells (Fig. 2): (i) tachyzoite (a form of rapid multiplication that is characteristically found in the acute infections); (ii) bradyzoite (a course of irksome multiplication that is characteristic of the chronic infection and that originates the tissue cysts; and (iii) sporozoite, which is produced only in the definitive host during the sexual reproduction and released in the oocysts via felid feces (Figs. one, two).
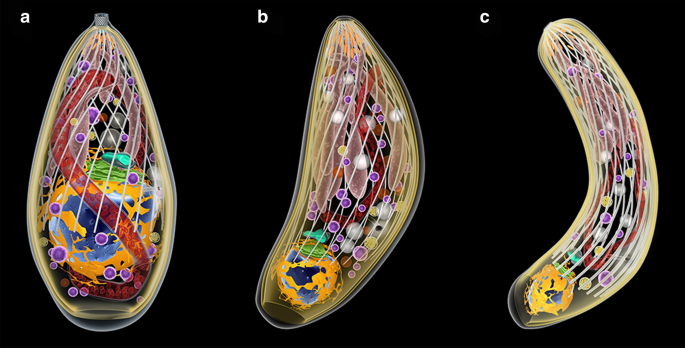
The 3 infective stages of T. gondii. Tachyzoite (a), bradyzoite (b), and sporozoite (c). The nucleus (blueish) is surrounded past the rough endoplasmic reticulum (yellow). To a higher place it, The Golgi complex (green) and the apicoplast (blue-green). The single mitochondrion spreads through the cytosol (red). Dense granules (magenta) and amylopectin granules (white) are dispersed in the cytosol. The apical circuitous is equanimous by the cylindrical conoid. Beneath, the secretory organelles: micronemes (orange) and rhoptries (pink). The cell trunk is express by three membrane units (the pellicle) and beneath it a prepare of subpelicular microtubules
The intermediate hosts tin can exist infected via dissimilar pathways including (i) ingestion of water, vegetables and fruits contaminated with viable oocysts, sporulated after previous emptying in the feces of cats (Fig. 1d); (two) intake of uncooked or undercooked meat containing viable tissue cysts (Fig. 1f); (iii) congenital transmission from the mother through the placenta (Fig. 1h); (4) blood transfusion (Fig. 1i); (v) organ transplantation, where the organs may contain cysts or tachyzoites (Fig.1j). Definitive hosts, i.e. felines, can be infected by carnivorism (both mammals and birds), or ingestion of sporulated oocysts. Oocysts can as well survive in oysters and mussels retaining its infectivity [30,31,32]. Ingestion of non-pasteurized milk or milk products is a potential source of transmission, although not common [8, 33].
Morphology and ultrastructure of the dissimilar forms of T. gondii
Infective forms (tachyzoites, bradyzoites and sporozoites)
Scanning and manual electron microscopy have been used to obtain a large number of images of the various developmental stages of T. gondii. The three infective forms (i.e. tachyzoite, bradyzoite and sporozoite) nowadays the same primary organization, displaying an elongated shape and a typical apical complex where structures and organelles such as the conoid, micronemes, and rhoptries are found (Fig. 2). Most of the available data is from tachyzoites (Fig. iii, Additional file i: Figures S1, Boosted file 2: Figures S2, Additional file 3: Figure S3) studied in more than detail [15, 20, 34,35,36,37,38,39,40,41,42].
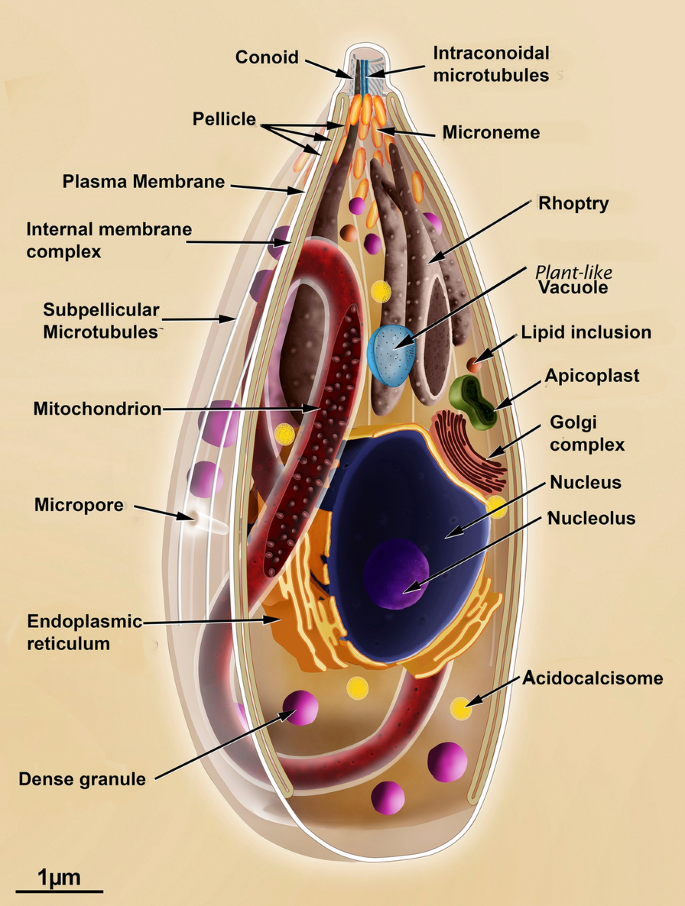
Longitudinal section view of the tachyzoite form of Toxoplasma gondii indicating the master structures and organelles
A complex of membranes known every bit the pellicle delimits the whole protozoan body. It is formed past an external plasma membrane and, below it, ii closely apposed membranes that form the inner membrane complex. This inner complex is absent from the most apical region where the conoid is located and in the about posterior part of the prison cell [43].
The three infective stages nowadays an explicit specialization of the anterior region where the upmost complex is localized. It is used to initiate the process of infection of host cells. This complex is formed by the conoid and ii sets of secretory organelles: the micronemes and the rhoptries [fifteen, 44].
The protozoan contains several structures that form its cytoskeleton. Below the inner membrane circuitous, at that place is a layer of microtubules. Twenty-two microtubules that originate at the polar band and project towards the posterior region of the cell, reaching about two thirds of the cell trunk length (Additional file 4: Figure S4). Besides, there is a network of filamentous structures with a mean diameter of viii–10 nm and are very labile and located immediately below the inner membrane complex [45]. They are equanimous of proteins of the articulin grouping called alveolins [46,47,48] and extend throughout the cell ending in a circular construction located at the posterior region, known as the basal complex, where proteins such equally the membrane occupation and recognition nexus exist (Morn-1) (Boosted file v: Figure S5) [35, 49].
1 characteristic feature of the T. gondii cytoskeleton is the conoid that appears as a hollow cylinder inserted within the polar ring from which the 22 sub-pellicular microtubules sally. It has a diameter of 400 nm and a length of 250 nm and appears in 2 states. In a resting state, it appears nether the polar band. When activated by an influx of Ca++, information technology extrudes towards the anterior region of the cells (Additional file 2: Effigy S2 and Additional file four: Figure S4) [16, 50]. This motion occurs during the procedure of host jail cell invasion. In the resting state, the conoid is positioned immediately below the plasma membrane, under the posterior band, from where the sub-pellicular microtubules emerge. 2 microtubules are situated inside the conoid, and 2 more apical rings are nowadays in its most upmost portion. The anterior ring has a bore of 160 nm, and the second measures 200 nm. Several proteins accept been shown to be in these circuitous structures, such as the calcium bounden proteins Centrin ii, TgCAM1, TgCAM2 [51] and TgMyoH myosin [52].
Another characteristic feature of the infective stages of T. gondii is the presence of the apical secretory organelles. Micronemes are the nearly arable ones (Fig. 3, Additional file i: Figure S1, Additional file 2: Effigy S2, Additional file iii: Figure S3, Boosted file 6: Figure S6). They appear as rod-like structures that are 250 nm long and 50 nm wide. They concentrate around the polar ring below the membrane system and seem to fuse with the region where only the plasma membrane exists [15, 53]. They are the first ones to secrete their poly peptide content, which is essential for the protozoan motion and its association with the membrane of the host cell [41]. The micronemal proteins are named MICs [54]. They include proteins with perforin-similar properties, adhesins, and serine proteases (subtilisins) [39, 55]. Some of the micronemal proteins are involved in the assembly (together with rhoptry proteins) of the moving junction [41, 56, 57]. Morphological analysis has shown that the number of micronemes is college in sporozoites, lower in bradyzoites, and intermediate in tachyzoites [3, 42]. Their secretion tin be induced by treatments that increase the intracellular calcium concentration [50, 58, 59].
Rhoptries comprise the second group of apical secretory organelles. They are larger than the micronemes, are lodge-shaped with two well-defined regions (Fig. 3, Additional file one: Figures S1, Additional file ii: Figure S2, Boosted file 3: Effigy S3, Additional file vi: Figure S6). The nearly basal one is wider and gives a spongy appearance, containing proteins involved in the subversion of host cell functions, known as ROPs. The anterior portion, or the neck, concentrates proteins associated with host prison cell invasion and are known as RONs. They present an acidic pH. Rhoptry secretion plays an essential role in the constitution of the moving junction for T. gondii invasion and formation of the parasitophorous vacuole (PV) membrane [sixty, 61].
The third group of secretory organelles are the dense granules (Additional file 6: Figure S6). They are commonly spherical with a hateful diameter of 0.ii μm and are distributed throughout the protozoan body. They besides contain a big number of proteins secreted at the lateral and posterior portion of the protozoan when it is localized inside the PV. The dense granule proteins are known every bit GRAs and are involved in the assembly of a network of tubules and filamentous structures with the PV. Their number is higher in sporozoites [fourteen, 15, 34, 62].
The nucleus of the protozoan is localized in the heart portion of the cell torso. It is roughly spherical, with a detached concavity at its upper side (i.e., the one that faces the apical complex) where the Golgi circuitous accommodates. During division, the nucleus assumes a horseshoe shape, maintaining the integrity of its membrane [63, 64].
Above the nucleus, the apicoplast is localized at a lateral position (Fig. iii, Additional file ii: Effigy S2, Additional file 3: Figure S3 and Additional file vii: Figure S7). The apicoplast is elongated and delimited by 4 membranes. It is an organelle of endosymbiotic origin and is closely associated with the Golgi complex and the endoplasmic reticulum. The apicoplast contains an extra chromosomal 35 kb DNA and the whole machinery that allows the synthesis of some proteins. It plays essential roles in biochemical processes such as blazon Two fatty acid synthesis and isoprenoid and Hemi grouping synthesis and is the first organelle to separate during the protozoan jail cell cycle [64,65,66].
Toxoplasma gondii presents profiles of the crude and shine endoplasmic reticulum distributed throughout the prison cell. The Golgi circuitous is closely apposed to the concave (anterior) portion of the nucleus, displaying 4 to half dozen lamellae. It divides at an early phase of the endodyogeny, and each complex is incorporated into one of the new cells formed [64, 67].
Toxoplasma gondii presents a single and ramified mitochondrion that tin reach nigh 10 µm long distributed throughout the prison cell [68, 69] (Fig. 3).
Toxoplasma gondii also contains nigh x acidocalcisomes, which are acidic organelles that store calcium and are involved in intracellular homeostasis and osmoregulation (Fig. 3). The diameters of these organelles vary from 40 to 150 nm and are dispersed in the cytoplasm [lxx].
Other cytoplasmic structures of T. gondii include lipid bodies, which seem to exist more than abundant in sporozoites (Additional file 8: Figure S8), and amylopectin granules (Additional file 9: Figure S9, Additional file 10: Figure S10, Additional file eleven: Figure S11), which are rarely seen in tachyzoites, just characteristically plant in bradyzoites (Additional file nine: Figure S9) and sporozoites. They appear equally spherical structures that contain reserve polysaccharides [iii].
Morphologically, bradyzoites and sporozoites differ from tachyzoites in several aspects, such every bit the position of the nucleus, that is more posterior in them; the number of micronemes, dense granules and amylopectin granules, as well as the aspect of the rhoptries (Additional files viii and 9: Figures S8 and S9) [42].
The life-wheel of T. gondii in the definitive host: schizonts, gametes and gametogenesis
Every bit previously mentioned, the sexual bike of T. gondii only takes place in members of the Felidae family. Most of the studies on this part of the cycle have been carried out in cats, especially in young kittens [27, 71, 72]. Due to the difficulties in maintaining and sacrifice cats in the laboratory, there are relatively few papers dealing with the bicycle of T. gondii in this host. The chief process of infection of a feline is by ingesting prey containing tissue cysts, or oocysts containing sporozoites excreted past some other feline (Fig. 1). Following ingestion, the wall of the cysts is disrupted in the stomach, probably due to its depression pH and the activity of proteolytic enzymes, releasing bradyzoites or sporozoites, respectively. In either situation, intestinal epithelial cells will be the first cells to be invaded and volition turn into schizonts, stage of asexual reproduction that tin can exist identified by the presence of several nuclei (Fig. 4).
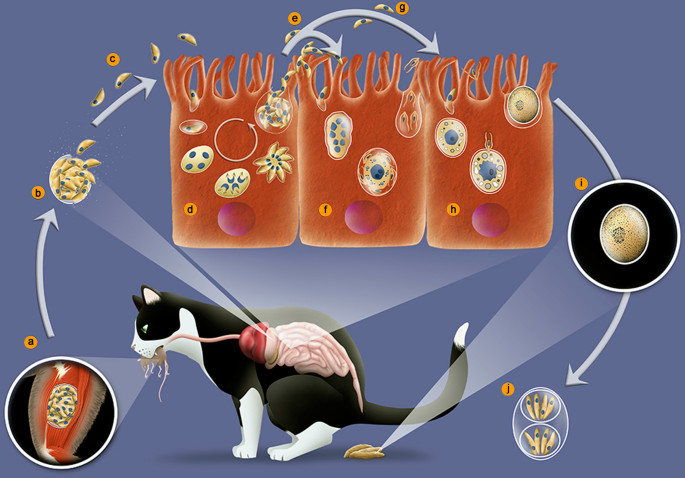
Life-cycle of Toxoplasma gondii in cat. a Ingestion of casualty containing tissue cysts. b The cyst wall is digested in the tummy and intestines, liberating bradyzoites. c Bradyzoites invade epitelial cells of the intestine. d In the enterocytes bradyzoites divide by schizogony giving rise to merozoites. e Merozoites differentiate into microgamonts, or macrogametes (f). g Fertilization gives rise to an unsporulated oocyst excreted with cat feces (h). i Sporulation occurs and generates 2 sporocysts with four sporozoites each (j)
During schizogony, successive nuclear divisions precede the individualization of each cell. Later on sequent rounds of nuclear division, the formation of the inner membrane complex for the individualization of each merozoite tin be followed in parallel with the upmost circuitous'southward appearance, including micronemes and rhoptries, all characteristic structures of a typical Apicomplexa. These cytoplasmic structures organize around each nucleus, giving rise to the girl cells, called merozoites inside the host cell. Rupture of these enterocytes releases many merozoites, which are as well able to infect new enterocytes and divide again by schizogony. Each schizogonic cycle gives rising several merozoites that will be released to readily invade new enterocytes, exponentially enhancing the number of parasites. Five different types of schizonts of T. gondii were described in feline intestinal epithelial cells earlier gametogony begins [73].
Three to xv days after the feline primary infection, the schizonts and merozoites are establish mainly in the ileum portion of the intestine, and some begin to differentiate into gametes (Fig. 4). Macrogametes are generated from a single merozoite and display an oval shape (8 µm long and 6 µm wide), a unmarried nucleus, endoplasmic reticulum, mitochondria, lipid bodies, amylopectin inclusions, and wall-forming bodies type I (diameter of 0.35 µm and electron dumbo) and II (width of 11.2 µm, less dumbo and in smaller number) (Fig. 5a). Microgametes are formed by a schizogonic division and are elongated (6 µm long and 2 µm wide); they display a condensed nuclear chromatin, ii basal bodies, respective flagella that are localized in the anterior region, and a mitochondrion that is restricted to the base of the flagella (Fig. 5b) [10, 62].
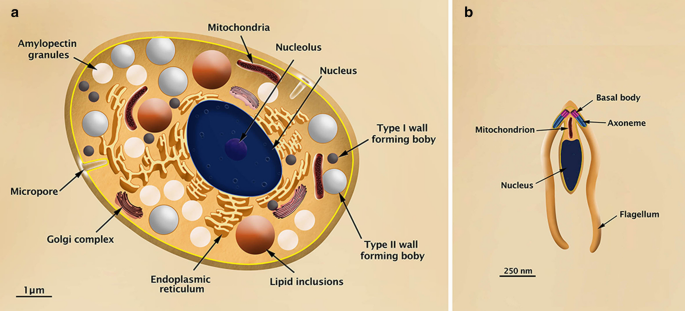
a Illustration of the macrogamete of Toxoplasma gondii in transversal section showing the main internal structures. b Analogy of the microgamete of Toxoplasma gondii in longitudinal section
The fusion of a microgamete with a macrogamete produces the immature oocyst, which is released into the intestinal lumen of the definitive host. The immature oocyst displays an elliptical shape (13 µm long × eleven µm broad) and contains in its interior a single sporoblast. Once the oocysts are liberated with the carrion of the cat, the aerated environment triggers maturation, i.e. sporulation. Upon sporulation, the oocyst divides into two sporocysts (vi–eight µm each). A thin and electron dense layer and an inner electron lucent layer as well as ii external membranes form the oocyst wall. After the subsequent oocyst maturation, each sporocyst contains 4 sporozoites. The sporulated oocysts are highly resistant to ecology conditions and remain viable either in h2o or in dry atmospheric condition for several months [3, 62, 74] (Boosted files 11: Figures S11, Additional file 12; Effigy S12, Boosted file thirteen: Effigy S13).
The life-bicycle of T. gondii in the intermediate host
Intermediate hosts tin be infected through several pathways (Fig. 1). Both tissue cyst and oocysts walls are removed by digestive enzymes, liberating, respectively, bradyzoites or sporozoites that within the new host, move by a unique mechanism of gliding [24, 56]. Micronemal proteins are the first to be secreted and are essential for protozoan motility by gliding and initial adhesion to the host cell surface. Gliding move results from a circuitous associates of proteins anchored to an actin myosin motor localized between the plasma membrane and the inner pellicle. This so-called glideosome involves microneme proteins (AMA1 and MIC2) inserted in the plasma membrane of the tachyzoite that recognize and attach to receptors of the host cell's plasma membrane. The intracellular domain of AM1 and MIC two are connected to an aldolase, that is linked to filamentous actin that is pushed by a TgMYO myosin motor and several GAP proteins (GAP40, GAP45, GAP50 and GAPM) that build a connection between the 3 membranes of the pellicle and the alveolin network [57]. This complex assembly pushes the tachyzoite forward towards a new host cell.
The interaction of T. gondii with a jail cell from the host aims, ultimately, its internalization. In T. gondii, this procedure can occur in whatsoever nucleated cell, particularly macrophages, epithelial cells, musculus cells, and neurons. Initially, T. gondii attaches with the surface of the potential host cell and in sequence reorients the apical side inducing, by secretion of proteins localized in the apical organelles, i.eastward. micronemes and rhoptries, the process of internalization (Fig. half dozen, Boosted file 14: Figure S14 and Additional file 15: Figure S15). For invasion, the tachyzoite assembles a moving junction with the plasma membrane of the host cell (Fig. 6, Additional file 15: Figure S15). This moving junction forms a band around the tachyzoite at the point of entry into the host cell. It results in the attachment of the micronemal protein AMA1 inserted in the surface of the tachyzoite to the rhoptry protein RON 2 that is secreted, inserted and exposed at the host cell membrane.
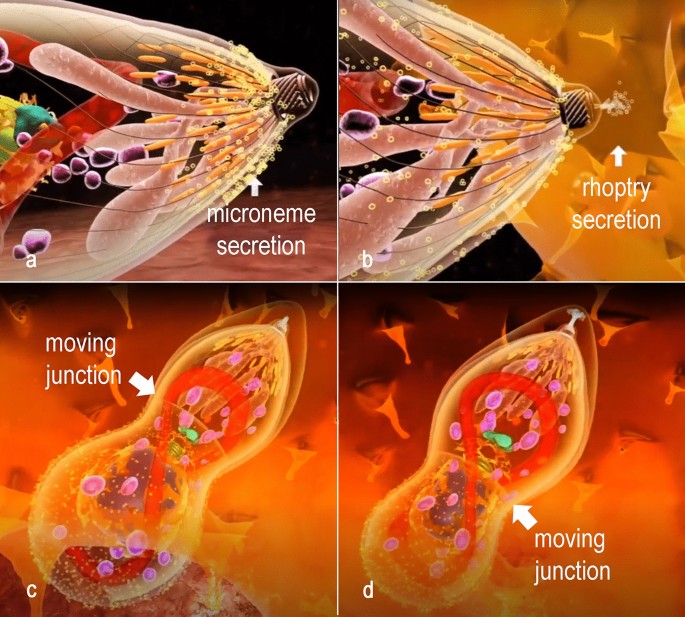
Sequential events of invasion of a host cell. a Microneme secretion and conoid extrusion are part of the gliding machinery. b The parasite attaches to the host jail cell membrane and secretion of proteins of the rhoptries neck induces host cell deformation. c, d A moving junction is established between the host cell and the parasite membrane at the point of invasion. Progressively the parasite invades the host jail cell protected in the PV, from which host cell proteins are excluded, equally well every bit proteins of the gliding machinery
Also RON two, the rhoptries also secrete RON 4, RON 5 and RON 8, which connect to RON2 in the cytoplasmic side of the host cell that associate with actin filaments. The tachyzoite squeezes itself along this moving junction, assuming an hourglass shape constricted at the point of contact (Fig. 6c, d). The process of parasite internalization is complex, and structures of the protozoan cytoskeleton (i.due east. the conoid) play a role as information technology moves up and downwards during the internalization procedure. In a further footstep, proteins in the basal portion of the rhoptry are secreted and implement modifications in the host cell beliefs and formation of the membrane of the parasitophorous vacuole where the protozoan volition survive and multiply (i.due east. the PV). Too, changes volition accept identify in the host cell cytosol. Amidst these modifications, nosotros point out the inhibition of fusion of host cell lysosomes with the PV membrane. On the other paw, the distribution of other organelles including the mitochondria and the endoplasmic reticulum tend to concentrate around the PV (Fig. 7). In contrast, the PV itself settles near the nucleus [75,76,77].
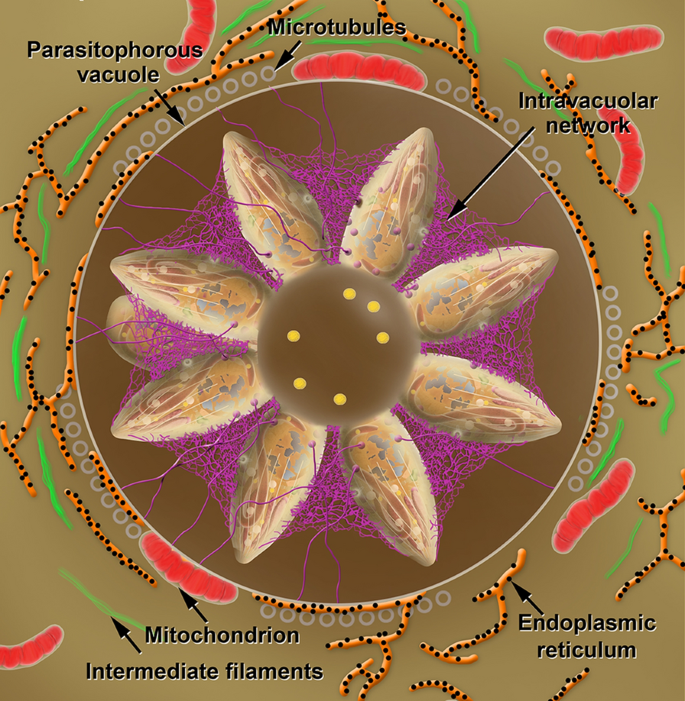
Schematic view of a PV in cantankerous section showing tachyzoites linked to the residue trunk. Inside the residual body, acidocalcisomes (yellow) are accumulated. An intravacuolar network (magenta) stabilizes the rosette of parasites. Profiles of the endoplasmic reticulum (orange) with ribosomes (blackness) adhered, microtubules (grey circles), intermediate filaments (greenish) and mitochondria (red) of the host cell and assemble around the PV
Inside the PV, secretion of proteins localized in the rhoptries and in dense granules occur, inducing modification of the PV membrane and the assembly of a network of tubular and filamentous structures within the PV (Fig. 7, Additional file 16: Figure S16). The tachyzoite starts a process of division past endodyogeny, where two daughter cells are assembled inside the mother cell. Successive divisions begin to create an organization of tachyzoites known every bit rosettes (Figs. 7, 8).
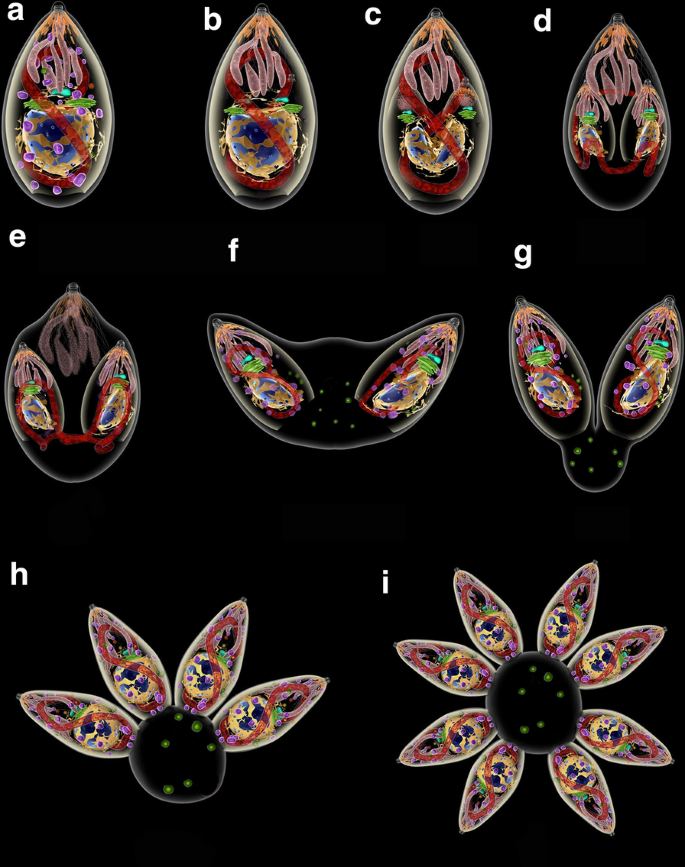
Sequence of events of division by endodiogeny. a, b The Golgi circuitous and the apicoplast are the first organelles to divide. c The nucleus assumes a horse-shoe shape. Two new apical complexes start to grade. d The inner pellicle grows and embraces the structures of the daughter cells, including the nucleus. e The mitochondrion is the last organelle to be separated between the girl cells. The upmost complex of the mother cell is all the same maintained at this bespeak. f The two daughter cells sally and the outer membrane of the female parent jail cell is incorporated. The apical complex of the mother cell disappears. g The two daughter cells remain linked to the residual trunk where acidocalcisomes (greenish) showtime to accrue. h The process is repeated until a rosette of parasites is formed (i)
After successive division cycles, some stimuli (e.g. a Ca++ influx) induce the egress of the tachyzoites and rupture the membrane of the host cell, liberating these new tachyzoites that tin can infect new host cells in the extracellular infinite (Additional file 17: Effigy S17) [50].
Inside a few days of infection, tachyzoites localized within a PV gradually begin to change their metabolism, slowing the division rate and converting into bradyzoites (Additional file eighteen: Figure S18). Cysts are more numerous in muscle cells and neurons than in other cell types. Under certain atmospheric condition including immunodepression, the bradyzoites can reconvert into tachyzoites.
Bradyzoites reside in tissue cysts. The size of each cyst varies co-ordinate to age, parasite strain and nature of the host cell. Pocket-size cysts have a bore of around 5 µm, while old cysts can accomplish 60 μm, and may contain about 2000 bradyzoites (Additional file 19: Effigy S19 and Boosted file 20: Figure S20).
The bradyzoites also secrete organelle contents into the PV matrix, which gradually lead to the assembly of a cyst wall in clan with the PV membrane and an intracystic network, every bit shown in Fig. 9a, b. The cyst wall is usually thin (< 0.5 µm) and formed past a limiting membrane, which is adjacent to a slightly electron dense matrix, and an inner layer where pocket-sized vesicles and tubules are seen.
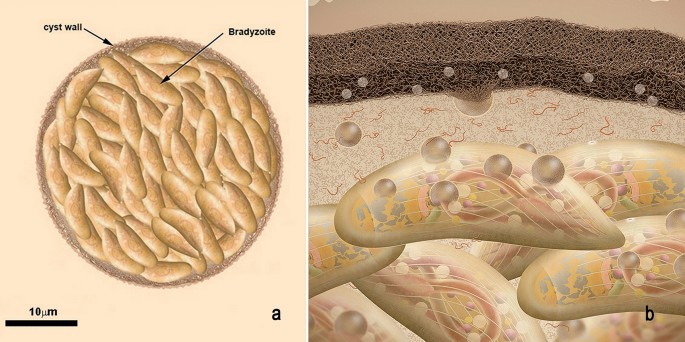
Scheme of a tissue cyst of Toxoplasma gondii. a The cyst wall is thick and filamentous. Each cyst may contain hundreds of bradyzoites. b A zoom view of the tissue cyst. The cyst is surrounded by a membrane and beneath information technology a thick cyst wall is deposited. The components of the cyst wall, too of the cyst matrix, are secreted past the bradyzoites
Animations
Office I. The cat as definitive host
A sequence of 2 animations was produced describing the life-bike of T. gondii in cat and man are included as Additional file 21: Video S1 and Additional files 22: Video S2, respectively. That was non a uncomplicated task, since there are many T. gondii manual paths between hosts, but also contaminated environment. We have made an pick to starting time with a brusque introductory text establishing T. gondii's medical importance. The action begins with a stray cat preying on a rat that was infected. In the digestive organisation of the cat, the bradyzoites are liberated from tissue cysts. Bradyzoites can invade any nucleated jail cell, simply similar tachyzoites and sporozoites. Nevertheless, enterocytes are probably the most attainable when infection occurs past carnivorism and are henceforth the preferential target cells for invasion. Inside hours of ingesting tissue cysts, a series of schizogonic cycles volition start in feline enterocytes, producing merozoites. 5 morphologically distinct types of schizonts were described [3, 62]. However, the number of schizogonic cycles that volition occur before gametogenesis starts is non known. So, for clarity, only one schizogonic cycle is represented in the animation. Another challenge was the representation of the gametogony, both micro and macrogametogenesis, raised many questions and doubts that are withal unanswered. A large number of microgametes are produced from a single merozoite. These are flagellated; hence, they are believed to be able to swim to actively find the macrogamete for fertilization. The macrogamete is produced from the differentiation of a single merozoite and is much larger than the microgamete and besides immotile. The blitheness of the process of fertilization event raised several questions. In principle, the microgametes released from a host jail cell would swim to find a host cell bearing a macrogamete to fertilize. All the same, how can the microgamete identify an enterocyte containing a macrogamete? Exercise trial and error brand it? More 1 PV is frequently found in a single host jail cell. Therefore, can fertilization occur from micro and macrogametes that originate in the aforementioned host cell? These hypotheses are discussed in the blitheness. Following fertilization, it is known that a considerable number of immature oocysts are released in the lumen of the gut and volition further be eliminated with the feces of the cat. Over again, we faced another unanswered question: since oocysts (too as macrogamonts) are immotile and relatively large (10–12 μm), how are they expelled from the host cell? A reasonable hypothesis is that immature oocysts are excreted as the enterocytes are discarded by apoptosis equally office of the usually intense renovation of the gut epithelium. The outset part of the blitheness ends at this point, with true cat feces containing oocysts being excreted and contaminating water and a vegetable garden.
Function Two. Animation of the interaction with the intermediate host
The second function of the blitheness starts by describing the sporulation procedure in the open air. Sporulated oocysts, containing two sporocysts with four sporozoites each, are highly infective when ingested with water of raw food (e.g. lettuce). Here, to avoid confusion and follow a direct forward path to exist clearly understood by the audience, the contamination of a new host past the ingestion of tissue cysts in uncooked meat is only mentioned, since it has been shown in the outset role of the animation. The breakdown of the oocyst and sporocyst walls during digestion is shown, followed by the invasion of host cells of the gut by the sporozoites. This bespeak is also cloudy, with unclear issues to be defined. The sporozoites are believed to rapidly convert to tachyzoites that will spread the parasites to other cells and organs.
A shut-up view of the tachyzoite gliding in the extracellular infinite allows the internal system of the protozoan to be observed, emphasizing the apical complex and secretion of micronemal proteins involved in gliding. Macrophages can act as efficient Trojan horses, transporting tacgyzoites, and for that reason, this is the model cell used in the blitheness. In the process of invasion, the secretion of micronemes, the motion of the conoid, the secretion from rhoptries, and the constriction characteristic of the moving junction between the plasma membranes of the tachyzoite and the host prison cell are emphasized. Afterwards invasion, the tachyzoites brainstorm to secrete the dense granules that will contribute to the enlargement of the membrane of the PV and generate a membranous network of tubules to continue the parasites stable inside the PV. The cycles of endodyogeny and the girl cells' zipper to the residual body result in the rosette assembly of tachyzoites [78]. Within the balance body, acidocalcisomes, which are represented by green spheres, accumulate. Upon egress, tachyzoites detach from the residual body and actively move towards the extracellular space, rupturing the host prison cell. Next, in the concluding sequence of the blitheness, the tachyzoites reach the central nervous organisation where conversion to bradyzoites occurs and tissue cysts develop. Information technology is well known that tissue cysts are besides formed in other tissues, every bit musculus and in the retina. Here this result is represented in a brain cell by a modify in the color of materials secreted by the parasites that turn from regal (tachyzoites) to green (bradyzoites). These materials accumulate within the vacuole and form the cyst wall. Bradyzoites are non organized as rosettes inside the cyst.
At this point there is a link with the first part of the blitheness, where the encephalon of the mouse contains tissue cysts, completing the cycle.
Conclusions
We tried to review the master aspects and establish a simplified sequence of events of a highly complex procedure: the life-cycle of T. gondii. We believe that, as well this text, the videos included in Additional files 21 and 22: Videos SV1 and SV2 and the PowerPoint® presentation of Additional file 23: Slideshow are excellent educational tools to exist used past teachers to discuss the several possible T. gondii transmission paths and basic information on the biology of the parasite. All these, resources are tools that may exist used by teachers both in traditional lectures and also to back up discussions after the exhibition of the movies. Access of online media for instruction and learning is a reality of our fourth dimension and, hopefully, this material will be beneficial for self-learning, or teaching, either in remote or presential fashion.
Availability of data and materials
Not applicable.
Abbreviations
- GRA:
-
Dense granule protein
- PV:
-
Parasitophorous vacuole
References
-
Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96.
-
Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;viii:634–40.
-
Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and evolution of tissue cysts. Clin Microbiol Rev. 1998;11:267–99.
-
CDC. Toxoplasmosis - epidemiology & hazard factors. Atlanta: Centers for Disease Control and Prevention; 2018. https://www.cdc.gov/parasites/toxoplasmosis/epi.html. Accessed 06 Aug 2020.
-
Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high brunt of affliction, and epidemiology. Parasitol. 2012;139:1375–424.
-
Tenter M, Heckeroth R, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–58.
-
Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. 2009;104:364–9.
-
Stelzer Southward, Basso W, Benavides Silván J, Ortega-Mora LM, Maksimov P, Gethmann J, Conraths FJ, Schares 1000. Toxoplasma gondii infection and toxoplasmosis in subcontract animals: chance factors and economic bear on. Food Waterborne Parasitol. 2019;xv:e00037.
-
Olsen A, Berg R, Tagel M, Must Thou, Deksne 1000, Enemark HL. Seroprevalence of Toxoplasma gondii in domestic pigs, sheep, cattle, wild boars, and moose in the Nordic-Baltic region: a systematic review and meta-assay. Parasite Epidemiol Control. 2019;5:e00100.
-
Frenkel JK, Dubey JP, Beattie CP. Toxoplasmosis of animals and man. J Parasitol. 2006;75:816.
-
Teixeira DE, Benchimol M, Rodrigues JCF, Crepaldi PH, Pimenta PF, de Souza W. The prison cell biological science of Leishmania: how to teach using animations. PLoS Pathog. 2013;9:e1003594.
-
Teixeira DE, Benchimol Thou, Crepaldi PH. de Souza Westward Interactive multimedia to teach the life bike of Trypanosoma cruzi, the causative amanuensis of Chagas disease. PLoS Negl Trop Dis. 2012;6:e1749.
-
Caldas LA, Attias One thousand, de Souza Westward. A structural analysis of the natural egress of Toxoplasma gondii. Microbes Infect. 2018;20:57–62.
-
Magno RCRC, Lemgruber Fifty, Vommaro RC, De Souza Westward, Attias M. Intravacuolar network may act as a mechanical back up for Toxoplasma gondii inside the parasitophorous vacuole. Microsc Res Tech. 2005;67:45–52.
-
Paredes-Santos TC, de Souza W, Attias M. Dynamics and 3D system of secretory organelles of Toxoplasma gondii. J Struct Biol. 2012;177:420–30.
-
Monteiro VG, De Melo EJT, Attias M, de Souza W. Morphological changes during conoid extrusion in Toxoplasma gondii tachyzoites treated with calcium ionophore. J Struct Biol. 2001;136:181–nine.
-
MacLaren A, Attias M, de Souza Due west. Aspects of the early on moments of interaction between tachyzoites of Toxoplasma gondii with neutrophils. Vet Parasitol. 2004;125:301–12.
-
De Souza West, Attias Chiliad. New advances in scanning microscopy and its application to study parasitic protozoa. Exp Parasitol. 2018;190:ten–33.
-
Attias M, Miranda Thou, De Souza West. Development and fate of the residual trunk of Toxoplasma gondii. Exp Parasitol. 2019;196:1–xi.
-
De Souza Due west, Attias M. New views of the Toxoplasma gondii parasitophorous vacuole as revealed by helium ion microscopy (HIM). J Struct Biol. 2015;191:76–85.
-
Grimwood J, Smith JE. Toxoplasma gondii: redistribution of tachyzoite surface protein during host jail cell invasion and intracellular development. Parasitol Res. 1995;81:657–61.
-
Dubremetz JF, Ferguson DJP. The role played by electron microscopy in advancing our understanding of Toxoplasma gondii and other apicomplexans. Int J Parasitol. 2009;39:883–93.
-
De Souza Due west. Fine structure of the conoid of Toxoplasma gondii. Rev Inst Med Trop Sao Paulo. 1974;16:32–8.
-
Frénal K, Dubremetz JF, Lebrun M, Soldati-Favre D. Gliding motility powers invasion and egress in Apicomplexa. Nat Rev Microbiol. 2017;xv:645–60.
-
Blader IJ, Coleman BI, Chen CT, Gubbels MJ. Lytic bicycle of Toxoplasma gondii: 15 years afterwards. Annu Rev Microbiol. 2015;69:463–85.
-
Horta MF, Andrade LO, Martins-Duarte ÉS, Castro-Gomes T. Prison cell invasion by intracellular parasites - the many roads to infection. J Cell Sci. 2020;133:jcs232488.
-
Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39:877–82.
-
Speer CA. Dubey JP Ultrastructure of schizonts and merozoites of Sarcocystis neurona. Vet Parasitol. 2001;95:263–71.
-
Martorelli Di Genova B, Wilson SK, Dubey JP, Knoll LJ. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol. 2019;17:e3000364.
-
Lindsay DS, Collins MV, Mitchell SM, Cole RA, Flick GJ, Wetch CN, et al. Survival of Toxoplasma gondii oocysts in eastern oysters (Crassostrea virginica). J Parasitol. 2004;90:1054–7.
-
Coupe A, Howe L, Shapiro K, Roe WD. Comparison of PCR assays to detect Toxoplasma gondii oocysts in light-green-lipped mussels (Perna canaliculus). Parasitol Res. 2019;118:2389–98.
-
Monteiro TRM, Rocha KS, Silva J, Mesquita GSS, Rosário MKS, Ferreira MFS, Honorio BET, et al. Detection of Toxoplasma gondii in Crassostrea spp. oysters cultured in an estuarine region in eastern Amazon. Zoonoses Public Wellness. 2019;66:296–300.
-
Chiari CA, Neves DP. Human toxoplasmosis acquired past ingestion of caprine animal's milk. Mem Inst Oswaldo Cruz. 1984;79:337–twoscore.
-
Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced past parasite proteins. Mol Biol Cell. 2002;xiii:2397–409.
-
Morrissette NS, Sibley LD. Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev. 2002;66:21–38.
-
Coppens I, Dunn JD, Romano JD, Marc P, Hui Z, John CB, et al. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Jail cell. 2006;125:261–74.
-
Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma jail cell invasion. Curr Opin Microbiol. 2007;10:83–9.
-
Coppens I, Romano JD. Hostile intruder: Toxoplasma holds host organelles convict. PLoS Pathog. 2018;xiv:e1006893.
-
Soldati D, Dubremetz JF, Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int J Parasitol. 2001;31:1293–302.
-
Magno RC, Straker LC, De Souza W, Attias M. Interrelations between the parasitophorous vacuole of Toxoplasma gondii and host cell organelles. Microsc Microanal. 2005;11:166–74.
-
Carruthers VB, Sibley LD. Sequential protein secretion from three singled-out organelles of Toxoplasma gondii accompanies invasion of homo fibroblasts. Eur J Cell Biol. 1997;73:114–23.
-
Speer CA, Clark S, Dubey JP. Ultrastructure of the oocysts, sporocysts, and sporozoites of Toxoplasma gondii. J Parasitol. 1998;84:505–12.
-
Porchet E, Torpier Thousand. Freeze fracture study of Toxoplasma and Sarcocystis infective stages. Z Parasitenkd. 1977;54:101–24.
-
Dubremetz JF. Host cell invasion by Toxoplasma gondii. Trends Microbiol. 1998;6:27–xxx.
-
Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–68.
-
Gould SB, Kraft LGK, van Dooren GG, Goodman CD, Ford KL, Cassin AM, et al. Ciliate pellicular proteome identifies novel protein families with feature echo motifs that are common to alveolates. Mol Biol Evol. 2001;28:1319–31.
-
Lemgruber L, Kloetzel JA, de Souza W, Vommaro RC. Toxoplasma gondii: further studies on the subpellicular network. Mem Inst Oswaldo Cruz. 2009;104:706–9.
-
Tosetti N, Dos Santos Pacheco N, Bertiaux Due east, Maco B, Bournonville L, Hamel 5, et al. Essential function of the alveolin network in the subpellicular microtubules and conoid assembly in Toxoplasma gondii. Elife. 2020;9:e56635.
-
Morrissette NS, Murray JM, Roos DS. Subpellicular microtubules acquaintance with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J Cell Sci. 1997;110:35–42.
-
Mondragon R, Frixione E. Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol. 1996;43:120–7.
-
Hu Chiliad, Johnson J, Florens L, Fraunholz M, Suravajjala South, DiLullo C, et al. Cytoskeletal components of an invasion machine - the upmost complex of Toxoplasma gondii. PLoS Pathog. 2006;2:e13.
-
Graindorge A, Frénal K, Jacot D, Salamun J, Marq JB, Soldati-Favre D. The conoid associated motor MyoH is indispensable for Toxoplasma gondii entry and exit from host cells. PLoS Pathog. 2016;12:e1005388.
-
Dubois DJ, Soldati-Favre D. Biogenesis and secretion of micronemes in Toxoplasma gondii. Prison cell Microbiol. 2019;21:e13018.
-
Carruthers VB, Tomley FM. Microneme proteins in apicomplexans. Subcell Biochem. 2008;47:33–45.
-
Kafsack BFC, Carruthers VB. Apicomplexan perforin-similar proteins. Commun Integr Biol. 2010;3:eighteen–23.
-
Opitz C, Soldati D. "The glideosome": a dynamic circuitous powering gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 2002;45:597–604.
-
Kato 1000. How does Toxoplasma gondii invade host cells? J Vet Med Sci. 2018;80:1702–6.
-
Hoff EF, Carruthers VB. Is Toxoplasma egress the first step in invasion? Trends Parasitol. 2002;18:251–5.
-
Lovett JL, Marchesini N, Moreno SNJ, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol ane,4,v-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem. 2002;277:25870–half-dozen.
-
Boothroyd JC, Dubremetz JFF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6:79–88.
-
Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10:582–vii.
-
Dubey JP. Toxoplasmosis. J Am Vet Med Assoc. 1986;189:166–70.
-
Hu K, Mann T, Striepen B, Beckers CJM, Roos DS, Murray JM. Daughter cell associates in the protozoan parasite Toxoplasma gondii. Mol Biol Prison cell. 2002;13:593–606.
-
Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121:1559–68.
-
Köhler S. Multi-membrane-bound structures of Apicomplexa: I. the architecture of the Toxoplasma gondii apicoplast. Parasitol Res. 2005;96:258–72.
-
Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, et al. A plastid of probable green algal origin in apicomplexan parasites. Science. 1987;275:1485–9.
-
Pelletier L, Stern CA, Pypaert M, Sheff D, Ngô HM, Roper N, et al. Golgi biogenesis in Toxoplasma gondii. Nature. 2002;418:548–52.
-
Tanabe Thou. Visualization of the mitochondria of Toxoplasma gondii-infected mouse fibroblasts past the cationic permeant fluorescent dye rhodamine 123. Experientia. 1985;41:101–2.
-
Melo EJLJ, Attias M, De Souza W. The single mitochondrion of tachyzoites of Toxoplasma gondii. J Struct Biol. 2000;130:27–33.
-
Rohloff P, Miranda K, Rodrigues JCF, Fang J, Galizzi M, Plattner H, Hentschel J, et al. Calcium uptake and proton transport past acidocalcisomes of Toxoplasma gondii. PLoS One. 2011;half-dozen:e18390.
-
Ferguson DJ, Hutchison WM, Dunachie JF, Siim JC. Ultrastructural study of early stages of asexual multiplication and microgametogony of Toxoplasma gondii in the small intestine of the cat. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974;82:167–81.
-
Hutchison WM, Dunachie JF, Piece of work Yard, Siim JC. The life cycle of the coccidian parasite, Toxoplasma gondii, in the domestic cat. Trans R Soc Trop Med Hyg. 1971;65:380–99.
-
Dubey JP, Frenkel JK. Cyst-induced toxoplasmosis in cats. J Protozool. 1972;nineteen:155–77.
-
Frenkel JK, Dubey JP, Miller NL. Toxoplasma gondii in cats: fecal stages identified every bit coccidian oocysts. Science. 1970;167:893–6.
-
Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Jail cell Sci. 1997;110:2117–28.
-
Romano JD, Coppens I. Hostorganelle hijackers: a similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: co-infection model equally a tool to investigate pathogenesis. Pathog Dis. 2013;69:72–86.
-
Wang Y, Weiss LM, Orlofsky A. Coordinate control of host centrosome position, organelle distribution, and migratory response by Toxoplasma gondii via host mTORC2. J Biol Chem. 2010;285:15611–8.
-
Muñiz-Hernández S, Carmen MG, Mondragón Thou, Mercier C, Cesbron MF, Mondragón-González SL, et al. Contribution of the rest trunk in the spatial organization of Toxoplasma gondii tachyzoites inside the parasitophorous vacuole. J Biomed Biotechnol. 2011;2011:473983.
Acknowledgements
This work was supported by grants from Brazilian Science Funding agencies: Conselho Nacional de Desenvolvimento Científico east Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado practise Rio de Janeiro (FAPERJ); Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB); and Instituto Nacional de Metrologia, Qualidade Industrial e Tecnologia (Inmetro). The authors are thankful to Paula Daros Almeida for technical support.
Funding
Grants to MA: Conselho Nacional de Desenvolvimento Cientifico (CNPq) grant process # 306745/2019-iv; and from Fundação Estadual Carlos Chagas Filho de Apoio à Pesquisa practise Rio de Janeiro (FAPERJ), # Due east-26/202.594/2019.
Author information
Authors and Affiliations
Contributions
MA fabricated substantial contributions to the design, analysis and interpretation of information and substantively revised it. DET made substantial contributions to the design of the piece of work. MB drafted and made substantial contributions to the conception of the work. RCV made meaning contributions to analysis and interpretation of data and substantively revised information technology. PHC fabricated substantial contributions to the design and cosmos of the animations. WDS made substantial contributions to the formulation of the piece of work and substantively revised information technology. All authors read and approved the concluding manuscript.
Respective writer
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Boosted information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Boosted file 21 : Video S1. Role 1- Life cycle of T. gondii in the feline host.
Additional file 22 : Video S2. Part two- Life wheel of T. gondii in the human being intermediate host.
Additional file iv: Effigy S4.
T. gondii cytoskeleton: Conoid (blackness arrow), polar ring (white pointer), subpellicular microtubules (arrowheads).
Additional file five: Figure S5.
Subpelicular network and microtubules. [47].
Boosted file 6: Figure S6.
Tachyzoite. Abbreviations: c, conoid; R, rhoptry; A, acidocalcisome; 1000, microneme; DG, dumbo granule.
Boosted file vii: Effigy S7.
Four Membranes of apicoplast (arrows). Inset, relative position of the apicoplast (A) to the nucleus (N) and Golgi complex (GC). (Image courtesy Dr. Erica Martins Duarte).
Additional file 10: Effigy S10
. Bradyzoite. Amylopectin granules (arrow).
Additional file 11: Effigy S11.
Sporocyst suture of curved plates (arrowheads).
Boosted file 12: Effigy S12.
3D scheme of a sporulated oocyst containing two sporocysts with 4 sporozoites each.
Additional file thirteen: Effigy S13.
Section view of a sporulated oocyst.
Additional file 14: Effigy S14.
Tachyzoite (imperial) adhered to a lymphocyte (beige) [17].
Additional file 15: Figure S15.
Tachyzoite (purple) invading a macrophage (beige).
Boosted file xvi: Figure S16.
Parasitophorous vacuole: rosette of tachyzoites (purple), filamentous network (pink). Host cell (beige)
Additional file 17: Figure S17.
Sequence of intracellular cycle. a Adhesion, secretion of ropthries. b Moving junction: T. gondii assumes an hourglass shape. c Secretion of dense granules inside the parasitophorous vacuole. d Division, formation of the intravacuolar network, aggregating of acidocalcisomes (green) in the balance body. e Rosette of parasites. f Individualization and egress of parasites.
Additional file xviii: Figure S18.
Cystogenesis. a Invasion. b establishment of parasitophorous vacuole. c Sectionalisation. d Bradyzoite secretion. e, f Cyst wall thickens, bradyzoites proceed to divide. g The cyst inside the host cell.
Boosted file xix: Figure S19.
Tissue cyst in the brain of a mouse. Bradyzoites (majestic) surrounded past a thick cyst wall (yellow). Blood vessel (cerise).
Additional file xx: Figure S20.
Tissue cyst. A cystwall (arrowhead). Amylopectin granules (asterisk), granular matrix (black star).
Additional file 23.
Slide show of T. gondii biological cycle, developmental stages and primary organelles.
Rights and permissions
Open Admission This article is licensed nether a Artistic Eatables Attribution 4.0 International License, which permits apply, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables licence, and indicate if changes were made. The images or other tertiary political party material in this article are included in the article's Artistic Commons licence, unless indicated otherwise in a credit line to the textile. If material is not included in the commodity'south Creative Eatables licence and your intended use is not permitted by statutory regulation or exceeds the permitted utilize, you volition need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/past/4.0/. The Artistic Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/ane.0/) applies to the information made available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
Nearly this commodity
Cite this article
Attias, M., Teixeira, D.E., Benchimol, M. et al. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors thirteen, 588 (2020). https://doi.org/x.1186/s13071-020-04445-z
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s13071-020-04445-z
Keywords
- Apicomplexa
- Parasitology
- Parasite
- Toxoplasmosis
- Protozoology
- Cell biology
- Life-bike
Source: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-020-04445-z
Posted by: bootsdoner1941.blogspot.com

0 Response to "What Animals Are Known To Be Intermediate Hosts For Toxoplasma"
Post a Comment